Microbially-Mediated Defluorination of High-Priority PFAS: Microorganisms, Genetics, and Biochemistry
Objective
This project will develop the scientific basis to allow for future applications of biological or coupled chemical-biological treatment processes to cost-effectively remove fluorotelomers and perfluorooctane sulfonate (PFOS) from subsurface environments. The specific objectives are to: (1) elucidate the mechanisms of bacterial desulfonation and metabolism of fluorotelomer sulfonates and sulfonamide derivatives at the genetic and biochemical levels; (2) improve understanding of step-wise microbial defluorination processes that lead to complete defluorination of fluorotelomers and characterize bacterial consortia and functional genes involved; and (3) develop an understanding of synergistic photochemical reduction and anaerobic microbial defluorination processes that degrade PFOS.
Benefits
This research will reduce DoD liabilities by establishing fundamental understanding of the microorganisms, genetics, and biochemistry involved in biological C-S and C-F cleavage. The knowledge will guide development of cost-effective biological treatment strategies in the long-term, while in the short-term will provide bacterial genetic markers for assessing the natural attenuation potential of microbial communities at DoD sites, monitoring the activity of those communities, and addressing site limitations inhibiting biological PFAS removal.
List of Publications:
2024
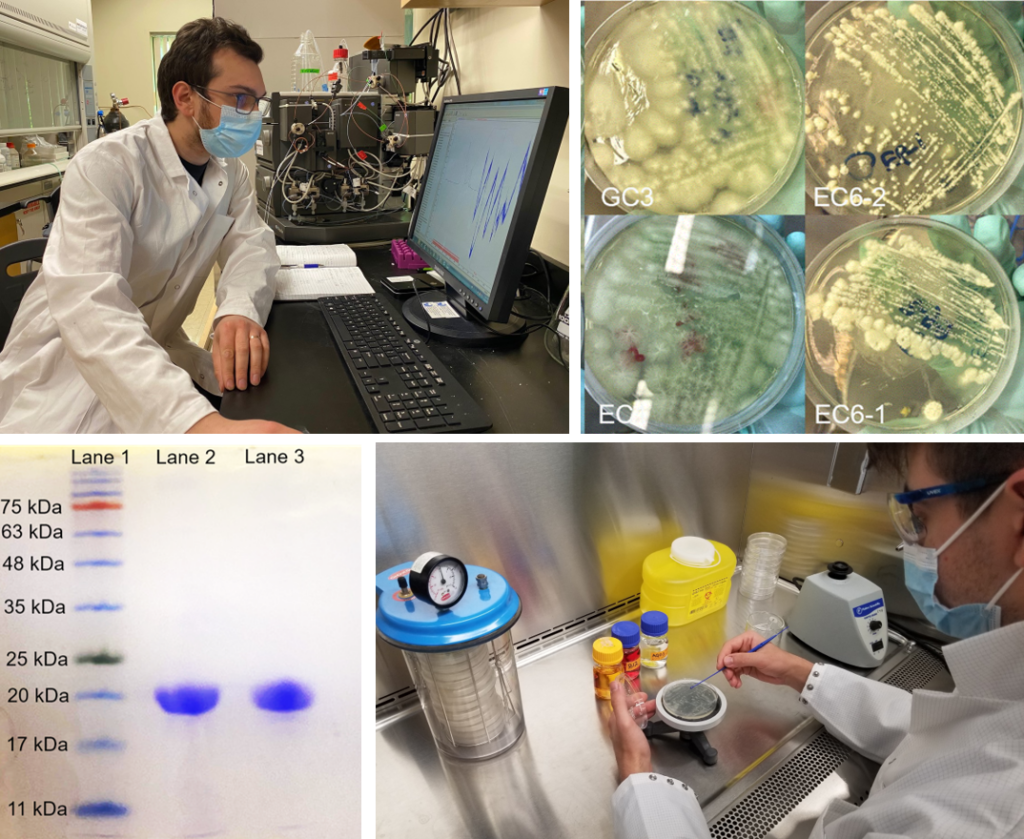
Per and polyfluoroalkyl substances (PFAS) are a large family of anthropogenic fluorinated chemicals of increasing environmental concern. Over recent years, numerous microbial communities have been found to be capable of metabolizing some polyfluoroalkyl substances, generating a range of low-molecular-weight PFAS metabolites. One proposed pathway for the microbial breakdown of fluorinated carboxylates includes β-oxidation, this pathway is initiated by the formation of a CoA adduct. However, until recently no PFAS-CoA adducts had been reported. In a previous study, we were able to use a bacterial medium-chain acyl-CoA synthetase (mACS) to form CoA adducts of fluorinated adducts of propanoic acid and pentanoic acid but were not able to detect any products of fluorinated hexanoic acid analogues. Herein, we expressed and purified a long-chain acyl-CoA synthetase (lACS) and a A461K variant of mACS from the soil bacterium Gordonia sp. strain NB4–1Y and performed an analysis of substrate scope and enzyme kinetics using fluorinated and nonfluorinated carboxylates. We determined that lACS can catalyze the formation of CoA adducts of 1:5 fluorotelomer carboxylic acid (FTCA), 2:4 FTCA and 3:3 FTCA, albeit with generally low turnover rates (<0.02 s–1) compared with the nonfluorinated hexanoic acid (5.39 s–1). In addition, the A461K variant was found to have an 8-fold increase in selectivity toward hexanoic acid compared with wild-type mACS, suggesting that Ala-461 has a mechanistic role in selectivity toward substrate chain length. This provides further evidence to validate the proposed activation step involving the formation of CoA adducts in the enzymatic breakdown of PFAS.
2023
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) make up a group of anthropogenic chemicals with a myriad of applications. However, some PFAS have been shown to negatively impact human health and the environment, leading to increased regulation, with some countries making efforts to phase out their use. PFAS fate in the environment is driven by physical, chemical, and biological processes, with microbial communities in matrices such as soil and sewage sludge being known to generate a range of low-molecular-weight PFAS metabolites. Proposed metabolic intermediates for both mixed and pure microbial cultures include fluorinated carboxylates that may be activated by CoA prior to β-oxidation and defluorination, although thus far, no PFAS-CoA adducts have been reported. Herein, we expressed and purified acyl-CoA synthetase (ACS) from the soil bacterium Gordonia sp. strain NB4-1Y and performed an analysis of substrate scope and enzyme kinetics using fluorinated and nonfluorinated carboxylates. We determined that ACS was able to catalyze the formation of CoA adducts of 3,3,3-trifluoropropionic acid, 5,5,5-trifluoropentanoic acid, 4,5,5-trifluoropent-4-enoic acid, and 4,4,5,5,5-pentafluoropentanoic acid. Kinetic analysis revealed a 90–98% decrease in kcat between nonfluorinated carboxylates and their fluorinated analogues. This provides evidence to validate proposed enzymatic pathways for microbial PFAS metabolism that proceed via an activation step involving the formation of CoA adducts.